Nanotechnology Development for Formulating Essential Oils in Wound Dressing Materials to Promote the Wound-Healing Process: A Review
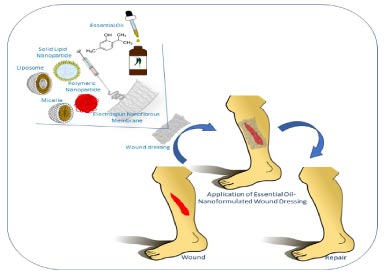
Wound healing refers to the replacement of damaged tissue through strongly coordinated
cellular events. The patient�s condition and different types of wounds complicate the already
intricate healing process. Conventional wound dressing materials seem to be insufficient to facilitate
and support this mechanism. Nanotechnology could provide the physicochemical properties and
specific biological responses needed to promote the healing process. For nanoparticulate dressing
design, growing interest has focused on natural biopolymers due to their biocompatibility and good
adaptability to technological needs. Polysaccharides are the most common natural biopolymers
used for wound-healing materials. In particular, alginate and chitosan polymers exhibit intrinsic
antibacterial and anti-inflammatory effects, useful for guaranteeing efficient treatment. Recent
studies highlight that several natural plant-derived molecules can influence healing stages. In
particular, essential oils show excellent antibacterial, antifungal, antioxidant, and antiinflammatory properties that can be amplified by combining them with nanotechnological
strategies. This review summarizes recent studies concerning essential oils as active secondary
compounds in polysaccharide-based wound dressings.
Bibliografia: - Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C 2015, 48, 651-662, doi:10.1016/j.msec.2014.12.068. - MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874, doi:10.1038/nature05664. - Bhardwaj, N.; Chouhan, D.; B Mandal, B. Tissue Engineered Skin and Wound Healing: Current Strategies and Future Directions. Curr. Pharm. Des. 2017, 23, 3455-3482, doi:10.2174/1381612823666170526094606. - Kapoor, M.; Appleton, I. Wound healing: Abnormalities and future therapeutic targets. Curr. Anaesth. Crit. Care 2005, 16, 88-93, doi:10.1016/j.cacc.2005.03.005. - Moura, L.I.F.; Dias, A.M.A.; Carvalho, E.; de Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093-7114, doi:10.1016/j.actbio.2013.03.033. - Cañedo-dorantes, L.; Cañedo-ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflamm. 2019, 2019, 3706315. - Sinno, H.; Prakash, S. Complements and the Wound Healing Cascade: An Updated Review. Plast. Surg. Int. 2013, 2013, 146764, doi:10.1155/2013/146764. - Tsala, D.E.; Dawe, A.; Habtemariam, S. Natural wound healing and bioactive natural products. Phytopharmacology 2013, 4, 532- 560. - Elnar, T.V.; Ailey, T.B. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med Res. 2009, 37, 1528-1542. - Stephens, P.; Thomas, D.W. The cellular proliferative phase of the wound repair process. J. Wound Care 2013, 11, doi:10.12968/jowc.2002.11.7.26421. - Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314, doi:10.1038/nature07039. - Bielefeld, K.A.; Amini-Nik, S.; Alman, B.A. Cutaneous wound healing: Recruiting developmental pathways for regeneration. Cell. Mol. Life Sci. 2013, 70, 2059-2081, doi:10.1007/s00018-012-1152-9. - Das, S.; Baker, A.B. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front. Bioeng. Biotechnol. 2016, 4, 82. Available online: https://www.frontiersin.org/article/10.3389/fbioe.2016.00082 (accessed on 22 November 2020). - Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; d'Ayala, G.G. Wound healing and Antimicrobial effect of active Secondary Metabolites in Chitosan-based Wound dressings: A review. Carbohydr. Polym. 2020, 115839, doi:10.1016/j.carbpol.2020.115839. - Suarato, G.; Bertorelli, R.; Athanassiou, A. Borrowing From Nature: Biopolymers and Biocomposites as Smart Wound Care Materials. Front. Bioeng. Biotechnol. 2018, 6, 137, doi:10.3389/fbioe.2018.00137. - Sikka, M.P.; Midha, V.K. The Role of Biopolymers and Biodegradable Polymeric Dressings in Managing Chronic Wounds, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019, doi:10.1016/b978-0-08-102192-7.00016-3. - Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511- 529, doi:10.1089/wound.2012.0401. - Naskar, A.; Kim, K.S. Recent advances in nanomaterial-based wound-healing therapeutics. Pharmaceutics 2020, 12, 499, doi:10.3390/pharmaceutics12060499. - Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857-2867, doi:10.1007/s11033-018-4296-3. - Tran, P.L.; Hamood, A.N.; de Souza, A.; Schultz, G.; Liesenfeld, B.; Mehta, D.; Reid, T.W. A study on the ability of quaternary ammonium groups attached to a polyurethane foam wound dressing to inhibit bacterial attachment and biofilm formation. Wound Repair Regen. 2015, 23, 74-81, doi:10.1111/wrr.12244. - Park, J.-H.; Choi, S.-H.; Park, S.-J.; Lee, Y.J.; Park, J.H.; Song, P.H.; Cho, C.-M.; Ku, S.-K.; Song, C.-H. Promoting Wound Healing Using Low Molecular Weight Fucoidan in a Full-Thickness Dermal Excision Rat Model. Mar. Drugs 2017, 15, 112, doi:10.3390/md15040112. - Gokarneshan, N. Review article-role of Chitosan in Wound Healing—A Review of the Recent Advances. Glob. J. Addic. Rehab. Med. 2017, 4, 555-636, doi:10.19080/GJARM.2017.04.555637. - Prosdocimi, M.; Bevilacqua, C. Exogenous hyaluronic acid and wound healing: An updated vision. Panminerva Med. 2012, 54, 129-135. - Straccia, M.C.; D'Ayala, G.G.; Romano, I.; Oliva, A.; Laurienzo, P. Alginate hydrogels coated with chitosan for wound dressing. Mar. Drugs 2015, 13, 2890-2908, doi:10.3390/md13052890. - Helary, C.; Abed, A.; Mosser, G.; Louedec, L.; Letourneur, D.; Coradin, T.; Giraud-Guille, M.M.; Meddahi-Pellé, A. Evaluation of dense collagen matrices as medicated wound dressing for the treatment of cutaneous chronic wounds. Biomater. Sci. 2015, 3, 373-382, doi:10.1039/C4BM00370E. - Zare-Gachi, M.; Daemi, H.; Mohammadi, J.; Baei, P.; Bazgir, F.; Hosseini-Salekdeh, S.; Baharvand, H. Improving anti-hemolytic, antibacterial and wound healing properties of alginate fibrous wound dressings by exchanging counter-cation for infected fullthickness skin wounds. Mater. Sci. Eng. C 2020, 107, 110321, doi:10.1016/j.msec.2019.110321. - Pacheco, M.S.; Kano, G.E.; Paulo, L.d.; Lopes, P.S.; de Moraes, M.A. Silk fibroin/chitosan/alginate multilayer membranes as a system for controlled drug release in wound healing. Int. J. Biol. Macromol. 2020, 152, 803-811, doi:10.1016/j.ijbiomac.2020.02.140. - Li, Y.; Chi, Y.-Q.; Yu, C.-H.; Xie, Y.; Xia, M.-Y.; Zhang, C.-L.; Han, X.; Peng, Q. Drug-free and non-crosslinked chitosan scaffolds with efficient antibacterial activity against both Gram-negative and Gram-positive bacteria. Carbohydr. Polym. 2020, 241, 116386, doi:10.1016/j.carbpol.2020.116386. - Hardy, A.; Seguin, C.; Brion, A.; Lavalle, P.; Schaaf, P.; Fournel, S.; Bourel-Bonnet, L.; Frisch, B.; de Giorgi, M. β-CyclodextrinFunctionalized Chitosan/Alginate Compact Polyelectrolyte Complexes (CoPECs) as Functional Biomaterials with AntiInflammatory Properties. ACS Appl. Mater. Interfaces. 2018, 10, 29347-29356, doi:10.1021/acsami.8b09733. - Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and AntiInflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1-13, doi:10.1155/2018/1708172. - Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421-430, doi:10.1016/j.jddst.2018.01.009. - Chou, S.F.; Gunaseelan, S.; Kiellani, M.H.H.; Thottempudi, V.V.K.; Neuenschwander, P.; Nie, H. A review of injectable and implantable biomaterials for treatment and repair of soft tissues in wound healing. J. Nanotechnol. 2017, 2017, 6341710, doi:10.1155/2017/6341710. - Moeini, A.; Masi, M.; Zonno, M.C.; Boari, A.; Cimmino, A.; Tarallo, O.; Vurro, M.; Evidente, A. Encapsulation of inuloxin A, a plant germacrane sesquiterpene with potential herbicidal activity, in β-cyclodextrins. Org. Biomol. Chem. 2019, 17, 2508-2515, doi:10.1039/C8OB03156H. - Johnson, J.L.; Raghavan, V.; Cimmino, A.; Moeini, A.; Petrovic, A.G.; Santoro, E.; Superchi, S.; Berova, N.; Evidente, A.; Polavarapu, P.L. Absolute configurations of chiral molecules with multiple stereogenic centers without prior knowledge of the relative configurations: A case study of inuloxin C. Chirality 2018, 30, 1206-1214, doi:10.1002/chir.23013. - Amrati, F.E.Z.; Bourhia, M.; Slighoua, M.; Ibnemoussa, S.; Bari, A.; Ullah, R.; Amaghnouje, A.; di Cristo, F.; el Mzibri, M.; Calarco, A.; et al. Phytochemical Study on Antioxidant and Antiproliferative Activities of Moroccan Caralluma europaea Extract and Its Bioactive Compound Classes. Evid.-Based Complement. Altern. Med. 2020, 2020, 8409718, doi:10.1155/2020/8409718. - Bahramsoltani, R.; Farzaei, M.H.; Rahimi, R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: An integrative review. Arch. Dermatol. Res. 2014, 306, 601-617, doi:10.1007/s00403-014-1474-6. - Kusumawati, I.; Indrayanto, G. Chapter 15—Natural Antioxidants in Cosmetics. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 485-505, doi:10.1016/B978-0-444-59603-1.00015-1. - Singh, G.; Kapoor, I.P.S.; Pandey, S.K.; Singh, U.K.; Singh, R.K. Studies on essential oils: Part 10; Antibacterial activity of volatile oils of some spices. Phyther. Res. 2002, 16, 680-682, doi:10.1002/ptr.951. - Houghton, P.J.; Hylands, P.J.; Mensah, A.Y.; Hensel, A.; Deters, A.M. In vitro tests and ethnopharmacological investigations: Wound healing as an example. J. Ethnopharmacol. 2005, 100, 100-107, doi:10.1016/j.jep.2005.07.001. - Minagawa, T.; Okamura, Y.; Shigemasa, Y.; Minami, S.; Okamoto, Y. Effects of molecular weight and deacetylation degree of chitin/chitosan on wound healing. Carbohydr. Polym. 2007, 67, 640-644, doi:10.1016/j.carbpol.2006.07.007. - Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.-M.; Helley, D.; ColliecJouault, S. Marine Polysaccharides: A Source of Bioactive Molecules for Cell Therapy and Tissue Engineering. Mar. Drugs 2011, 9, 1664-1681, doi:10.3390/md9091664. - Amaghnouje, A.; Mechchate, H.; Es-Safi, I.; Alotaibi, A.A.; Noman, O.M.; Nasr, F.A.; Al-Zharani, M.; Cerruti, P.; Calarco, A.; el Fatemi, H.; et al. Anxiolytic, antidepressant-like proprieties and impact on the memory of the hydro-ethanolic extract of origanum majorana L. On mice. Appl. Sci. 2020, 10, 8420, doi:10.3390/app10238420. - Amaghnouje, A.; Mechchate, H.; Es-safi, I.; Boukhira, S.; Aliqahtani, A.S.; Noman, O.M.; Nasr, F.A.; Conte, R.; Calarco, A.; Bousta, D. Subacute Assessment of the Toxicity and Antidepressant-Like Effects of Origanum Majorana L. Polyphenols in Swiss Albino Mice. Molecules 2020, 25, 5653, doi:10.3390/molecules25235653. - Masi, M.; Moeini, S.A.; Boari, A.; Cimmino, A.; Vurro, M.; Evidente, A. Development of a rapid and sensitive HPLC method for the identification and quantification of cavoxin and cavoxone in Phoma cava culture filtrates. Nat. Prod. Res. 2017, 6419, 1-5, doi:10.1080/14786419.2017.1392950. - Marturano, V.; Bizzarro, V.; Ambrogi, V.; Cutignano, A.; Tommonaro, G.; Abbamondi, G.R.; Giamberini, M.; Tylkowski, B.; Carfagna, C.; Cerruti, P. Light-responsive nanocapsule-coated polymer films for antimicrobial active packaging. Polymers 2019, 11, 68, doi:10.3390/polym11010068. - Marturano, V.; Marcille, H.; Cerruti, P.; Bandeira, N.A.G.; Giamberini, M.; Trojanowska, A.; Tylkowski, B.; Carfagna, C.; Ausanio, G.; Ambrogi, V. Visible-Light Responsive Nanocapsules for Wavelength-Selective Release of Natural Active Agents. ACS Appl. Nano Mater. 2019, 2, 4499-4506, doi:10.1021/acsanm.9b00882. - Moeini, A.; van Reenen, A.; van Otterlo, W.; Cimmino, A.; Masi, M.; Lavermicocca, P.; Valerio, F.; Immirzi, B.; Santagata, G.; Malinconico, M.; et al. α-costic acid, a plant sesquiterpenoid from Dittrichia viscosa, as modifier of Poly (lactic acid) properties: A novel exploitation of the autochthone biomass metabolite for a wholly biodegradable system. Ind. Crops Prod. 2020, 146, 112134, doi:10.1016/j.indcrop.2020.112134. - Moeini, A.; Mallardo, S.; Cimmino, A.; Poggetto, G.D.; Masi, M.; di Biase, M.; van Reenen, A.; Lavermicocca, P.; Valerio, F.; Evidente, A.; et al. Thermoplastic starch and bioactive chitosan sub-microparticle biocomposites: Antifungal and chemicophysical properties of the films. Carbohydr. Polym. 2020, 230, 115627, doi:10.1016/j.carbpol.2019.115627. - Moeini, A.; Cimmino, A.; Masi, M.; Evidente, A.; van Reenen, A. The incorporation and release of ungeremine, an antifungal Amaryllidaceae alkaloid, in poly(lactic acid)/poly(ethylene glycol) nanofibers. J. Appl. Polym. Sci. 2020, 137, 49098, doi:10.1002/app.49098. - Moeini, A.; Cimmino, A.; Poggetto, G.D.; di Biase, M.; Evidente, A.; Masi, M.; Lavermicocca, P.; Valerio, F.; Leone, A.; Santagata, G.; et al. Effect of pH and TPP concentration on chemico-physical properties, release kinetics and antifungal activity of ChitosanTPP-Ungeremine microbeads. Carbohydr. Polym. 2018, 195, 631-641, doi:10.1016/j.carbpol.2018.05.005. - Valerio, F.; Masi, M.; Cimmino, A.; Moeini, S.A.; Lavermicocca, P.; Evidente, A. Antimould microbial and plant metabolites with potential use in intelligent food packaging. Nat. Prod. Res. 2017, 6419, 1-6, doi:10.1080/14786419.2017.1385018. - Kumar, L.; Brice, J.; Toberer, L.; Klein-Seetharaman, J.; Knauss, D.; Sarkar, S.K. Antimicrobial biopolymer formation from sodium alginate and algae extract using aminoglycosides. PLoS ONE. 2019, 14, e0214411, doi:10.1371/journal.pone.0214411. - Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25-49, doi:10.1016/j.actbio.2020.02.022. - Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of Silk Fibroin Use in Wound Dressings. Trends Biotechnol. 2018, 36, 907-922, doi:10.1016/j.tibtech.2018.04.004. - Boateng, J.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review. J. Pharm. Sci. 2015, 104, 3653-3680, doi:10.1002/jps.24610. - Harrison, I.P.; Spada, F. Hydrogels for Atopic Dermatitis and Wound Management: A Superior Drug Delivery Vehicle. Pharmaceutics 2018, 10, 71, doi:10.3390/pharmaceutics10020071. - Chen, H.; Cheng, R.; Zhao, X.; Zhang, Y.; Tam, A.; Yan, Y.; Shen, H.; Zhang, Y.S.; Qi, J.; Feng, Y.; et al. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019, 11, 3, doi:10.1038/s41427-018-0103-9. - Xue, Z.; Wang, S.; Lin, L.; Chen, L.; Liu, M.; Feng, L.; Jiang, L. A Novel Superhydrophilic and Underwater Superoleophobic Hydrogel-Coated Mesh for Oil/Water Separation. Adv. Mater. 2011, 23, 4270-4273, doi:10.1002/adma.201102616. - Namazi, H.; Rakhshaei, R.; Hamishehkar, H.; Kafil, H.S. Antibiotic loaded carboxymethylcellulose/MCM-41 nanocomposite hydrogel films as potential wound dressing. Int. J. Biol. Macromol. 2016, 85, 327-334, doi:10.1016/j.ijbiomac.2015.12.076. - Zhang, D.; Zhou, W.; Wei, B.; Wang, X.; Tang, R.; Nie, J.; Wang, J. Carboxyl-modified poly(vinyl alcohol)-crosslinked chitosan hydrogel films for potential wound dressing. Carbohydr. Polym. 2015, 125, 189-199, doi:10.1016/j.carbpol.2015.02.034. - Monjezi, J.; Jamaledin, R.; Ghaemy, M.; Moeini, A.; Makvandi, P. A Performance Comparison of Graft Copolymer Hydrogels Based on Functionalized-Tragacanth Gum/Polyacrylic Acid and Polyacrylamide as Antibacterial and Antifungal Drug Release Vehicles. Am. J. Nanotechnol. Nanomed. Res. 2018, 1, 010-015. - Singh, R.; Singh, D. Radiation synthesis of PVP/alginate hydrogel containing nanosilver as wound dressing. J. Mater. Sci. Mater. Med. 2012, 23, 2649-2658, doi:10.1007/s10856-012-4730-3. - Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVAbased hydrogel dressings. J. Adv. Res. 2017, 8, 217-233, doi:10.1016/j.jare.2017.01.005. - Makvandi, P.; Gu, J.t.; Zare, E.N.; Ashtari, K.; Moeini, A.; Tay, F.R.; Niu, L. Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry. Acta Biomater. 2020, 101, 69-101, doi:10.1016/j.actbio.2019.09.025. - Stashak, T.S.; Farstvedt, E.; Othic, A. Update on wound dressings: Indications and best use. Clin. Tech. Equine Pract. 2004, 3, 148- 163, doi:10.1053/j.ctep.2004.08.006. - Jayakumar, R.; Prabaharan, M.; Kumar, P.T.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322-337, doi:10.1016/j.biotechadv.2011.01.005. - Lin, W.-C.; Lien, C.-C.; Yeh, H.-J.; Yu, C.-M.; Hsu, S. Bacterial cellulose and bacterial cellulose-chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603-611, doi:10.1016/j.carbpol.2013.01.076. - Sezer, A.D.; Cevher, E. Biopolymers as Wound Healing Materials: Challenges and New Strategies. Biomater. Appl. Nanomed. 2011, 383-414, doi:10.5772/25177. - Bianchera, A.; Catanzano, O.; Boateng, J.; Elviri, L. The Place of Biomaterials in Wound Healing. Ther. Dressings Wound Heal. Appl. 2020, 337-366, doi:10.1002/9781119433316.ch15. - Nesic, A.; Moeini, A.; Santagata, G. Marine biopolymers: Alginate and chitosan. Sustain. Polym. Mater. 2020, 73, doi:10.1515/9783110590586-004. - Huang, S.; Fu, X. Naturally derived materials-based cell and drug delivery systems in skin regeneration. J. Control. Release. 2010, 142, 149-159, doi:10.1016/j.jconrel.2009.10.018. - Hakkarainen, T.; Koivuniemi, R.; Kosonen, M.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Vuola, J.; Valtonen, J.; Tammela, P.; Mäkitie, A.; Luukko, K.; et al. Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J. Control. Release. 2016, 244, 292-301, doi:10.1016/j.jconrel.2016.07.053. - Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137-148, doi:10.1016/j.ijbiomac.2018.10.120. - Tayeb, A.H.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose nanomaterials-binding properties and applications: A review. Molecule 2018, 23, 2684, doi:10.3390/molecules23102684. - Yadav, T.C.; Srivastava, A.K.; Raghuwanshi, N.; Kumar, N.; Prasad, R.; Pruthi, V. Wound Healing Potential of Natural Polymer: Chitosan “A Wonder Molecule”. Integr. Green Chem. Sustain. Eng. 2019, 527-579, doi:10.1002/9781119509868.ch16. - Mohan, S.; Oluwafemi, O.S.; Kalarikkal, N.; Thomas, S.; Songca, S.P. Biopolymers—Application in Nanoscience and Nanotechnology. Recent Adv. Biopolym. 2016, 1, 47-66, doi:10.5772/62225. - Weller, C.D.; Team, V.; Sussman, G. First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence. Front. Pharmacol. 2020, 11, 155, doi:10.3389/fphar.2020.00155. - Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017, 3, 163-175, doi:10.1021/acscentsci.6b00371. - Zarrintaj, P.; Moghaddam, A.S.; Manouchehri, S.; Atoufi, Z.; Amiri, A.; Amirkhani, M.A.; Nilforoushzadeh, M.A.; Saeb, M.R.; Hamblin, M.R.; Mozafari, M. Can regenerative medicine and nanotechnology combine to heal wounds? the search for the ideal wound dressing. Nanomedicine 2017, 12, 2403-2422, doi:10.2217/nnm-2017-0173. - Berthet, M.; Gauthier, Y.; Lacroix, C.; Verrier, B.; Monge, C. Nanoparticle-Based Dressing: The Future of Wound Treatment? Trends Biotechnol. 2017, 35, 770-784, doi:10.1016/j.tibtech.2017.05.005. - Bhattacharya, D.; Ghosh, B.; Mukhopadhyay, M. Development of nanotechnology for advancement and application in wound healing: A review. IET Nanobiotechnology. 2019, 13, 778-785, doi:10.1049/iet-nbt.2018.5312. - Du, J.; Wong, K.K.Y. Nanomaterials for Wound Healing: Scope and Advances; Elsevier Inc.: Amsterdam, The Netherlands, 2019, doi:10.1016/B978-0-12-815341-3.00009-2. - Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release. 2017, 252, 28-49, doi:10.1016/j.jconrel.2017.03.008. - Niska, K.; Zielinska, E.; Radomski, M.W.; Inkielewicz-Stepniak, I. Metal nanoparticles in dermatology and cosmetology: Interactions with human skin cells. Chem. Biol. Interact. 2018, 295, 38-51, doi:10.1016/j.cbi.2017.06.018. - Ouyang, S.; Hu, X.; Zhou, Q.; Li, X.; Miao, X.; Zhou, R. Nanocolloids in Natural Water: Isolation, Characterization, and Toxicity. Environ. Sci. Technol. 2018, 52, 4850-4860, doi:10.1021/acs.est.7b05364. - Korrapati, P.S.; Karthikeyan, K.; Satish, A.; Krishnaswamy, V.R.; Venugopal, J.R.; Ramakrishna, S. Recent advancements in nanotechnological strategies in selection, design and delivery of biomolecules for skin regeneration. Mater. Sci. Eng. C 2016, 67, 747-765, doi:10.1016/j.msec.2016.05.074. - Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149-173, doi:10.1146/annurev-chembioeng-073009-100847. - Zhang, P.; He, L.; Zhang, J.; Mei, X.; Zhang, Y.; Tian, H.; Chen, Z. Preparation of novel berberine nano-colloids for improving wound healing of diabetic rats by acting Sirt1/NF-κB pathway. Colloids Surfaces B Biointerfaces. 2020, 187, 110647, doi:10.1016/j.colsurfb.2019.110647. - Sen, C.K. Wound healing essentials: Let there be oxygen. Wound Repair Regen. 2009, 17, 1-18, doi:10.1111/j.1524- 475X.2008.00436.x. - Ashter, S.A. Thermoforming of Single and Multilayer Laminates: Plastic Films Technologies, Testing, and Applications, Thermoforming Single Multilayer Laminates Plastic Films Technologies. Testing Appl. 2014, 123-145. - Chakrabarti, S.; Chattopadhyay, P.; Islam, J.; Ray, S.; Raju, P.S.; Mazumder, B. Aspects of Nanomaterials in Wound Healing. Curr. Drug Deliv. 2019, 16, 26-41, doi:10.2174/1567201815666180918110134. - Lei, J.; Sun, L.; Li, P.; Zhu, C.; Lin, Z. The Wound Dressings and Their Applications in Wound Healing and Management. Heal. Sci. J. 2019, 13, 1-8. Available online: http://www.imedpub.com/ (accessed on 22 November 2020). - Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based. Complement. Alternat. Med. 2014, 2014, 651593, doi:10.1155/2014/651593. - Echeverría, J.; de Albuquerque, R.D.D.G. Nanoemulsions of Essential Oils: New Tool for Control of Vector-Borne Diseases and In Vitro Effects on Some Parasitic Agents. Medicines 2019, 6, 42, doi:10.3390/medicines6020042. - Shakeel, F.; Faisal, M.S. Nanoemulsion: A promising tool for solubility and dissolution enhancement of celecoxib. Pharm. Dev. Technol. 2010, 15, 53-56, doi:10.3109/10837450902967954. - Azmi, N.A.; Elgharbawy, A.A.M.; Motlagh, S.R.; Samsudin, N.; Salleh, H.M. Nanoemulsions: Factory for food, pharmaceutical and cosmetics. Processes 2019, 7, 617, doi:10.3390/pr7090617. - Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter. 2016, 12, 2826-2841, doi:10.1039/C5SM02958A. - Simonazzi, A.; Cid, A.G.; Villegas, M.; Romero, A.I.; Palma, S.D.; Bermúdez, J.M. Chapter 3—Nanotechnology Applications in Drug Controlled Release. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 81-116, doi:10.1016/B978-0-12-813689-8.00003-3. - Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102-110, doi:10.1016/j.cocis.2005.06.004. - Sayed, S. Essential Oil Nanoformulations as a Novel Method for Insect Pest Control in Horticulture. In Horticultural Crops; IntechOpen: London, UK, 2019, doi:10.5772/intechopen.80747. - Abreu, F.S.; Costa, E.F.; Cardial, M.L.; André, W.P.P. Polymeric nanoemulsions enriched with Eucalyptus citriodora essential oil. Polímeros 2020, 30, doi:10.1590/0104-1428.00920. - Flores, F.; Lima, J.; Silva, C.; Benvegnú, D.; Ferreira, J.; Bürger, M.; Beck, R.; Rolim, C.; Rocha, M.; Veiga, M.; et al. Hydrogels Containing Nanocapsules and Nanoemulsions of Tea Tree Oil Provide Antiedematogenic Effect and Improved Skin Wound Healing. J. Nanosci. Nanotechnol. 2015, 15, 800-809, doi:10.1166/jnn.2015.9176. - Hamedi, H.; Moradi, S.; Tonelli, A.E.; Hudson, S.M. Preparation and Characterization of Chitosan-Alginate Polyelectrolyte Complexes Loaded with Antibacterial Thyme Oil Nanoemulsions. Appl. Sci. 2019, 9, 3933, doi:10.3390/app9183933. - Wang, F.; Hu, S.; Jia, Q.; Zhang, L. Advances in Electrospinning of Natural Biomaterials for Wound Dressing. J. Nanomater. 2020, 2020, 8719859, doi:10.1155/2020/8719859. - Bombin, A.D.J.; Dunne, N.J.; McCarthy, H.O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater. Sci. Eng. C 2020, 114, 110994, doi:10.1016/j.msec.2020.110994. - Memic, A.; Abudula, T.; Mohammed, H.S.; Navare, K.J.; Colombani, T.; Bencherif, S.A. Latest Progress in Electrospun Nanofibers for Wound Healing Applications. ACS Appl. Bio Mater. 2019, 2, 952-969, doi:10.1021/acsabm.8b00637. - Mele, E. Electrospinning of Essential Oils. Polymers 2020, 12, 908, doi:10.3390/polym12040908. - Cai, H.; Li, G. Efficacy of alginate-and chitosan-based scaffolds on the healing of diabetic skin wounds in animal experimental models and cell studies: A systematic review. Wound Repair Regen. 2020, 28, 751-771, doi:10.1111/wrr.12857. - Qin, X. Coaxial electrospinning of nanofibers. In Electrospun Nanofibers; Woodhead Publishing: Oxford, UK, 2017; pp. 41-71, doi:10.1016/B978-0-08-100907-9.00003-9. - Zhu, L.; Liu, X.; Du, L.; Jin, Y. Preparation of asiaticoside-loaded coaxially electrospinning nanofibers and their effect on deep partial-thickness burn injury. Biomed. Pharmacother. 2016, 83, 33-40, doi:10.1016/j.biopha.2016.06.016. - Pawar, H.V.; Tetteh, J.; Boateng, J.S. Preparation, optimisation and characterisation of novel wound healing film dressings loaded with streptomycin and diclofenac. Colloids Surfaces B Biointerfaces. 2013, 102, 102-110, doi:10.1016/j.colsurfb.2012.08.014. - Sabitha, M.; Rajiv, S. Preparation and characterization of ampicillin-incorporated electrospun polyurethane scaffolds for wound healing and infection control. Polym. Eng. Sci. 2015, 55, 541-548, doi:10.1002/pen.23917. - Lan, Y.; Li, W.; Guo, R.; Zhang, Y.; Xue, W.; Zhang, Y. Preparation and characterisation of vancomycin-impregnated gelatin microspheres/silk fibroin scaffold. J. Biomater. Sci. Polym. Ed. 2014, 25, 75-87, doi:10.1080/09205063.2013.836951. - Pásztor, N.; Rédai, E.; Szabó, Z.-I.; Sipos, E. Preparation and Characterization of Levofloxacin-Loaded Nanofibers as Potential Wound Dressings. Acta Medica Marisiensis. 2017, 63, 66-69, doi:10.1515/amma-2017-0014. - Mohseni, M.; Shamloo, A.; Aghababaei, Z.; Vossoughi, M.; Moravvej, H. Antimicrobial Wound Dressing Containing Silver Sulfadiazine With High Biocompatibility: In Vitro Study. Artif. Organs. 2016, 40, 765-773, doi:10.1111/aor.12682. - Adhirajan, N.; Shanmugasundaram, N.; Shanmuganathan, S.; Babu, M. Collagen-based wound dressing for doxycycline delivery: In-vivo evaluation in an infected excisional wound model in rats. J. Pharm. Pharmacol. 2009, 61, 1617-1623, doi:10.1211/jpp.61.12.0005. - Shao, W.; Liu, H.; Wang, S.; Wu, J.; Huang, M.; Min, H.; Liu, X. Controlled release and antibacterial activity of tetracycline hydrochloride-loaded bacterial cellulose composite membranes. Carbohydr. Polym. 2016, 145, 114-120, doi:10.1016/j.carbpol.2016.02.065. - Schultz, G.S.; Sibbald, R.G.; Falanga, V.; Ayello, E.A.; Dowsett, C.; Harding, K.; Romanelli, M.; Stacey, M.C.; Teot, L.; Vanscheidt, W. Wound bed preparation: A systematic approach to wound management. Wound Repair Regen. 2003, 11, S1-S28, doi:10.1046/j.1524-475X.11.s2.1.x. - Zhang, Q.; Fong, C.C.; Yu, W.K.; Chen, Y.; Wei, F.; Koon, C.M.; Lau, K.M.; Leung, P.C.; Lau, C.B.S.; Fung, K.P.; et al. Herbal formula Astragali Radix and Rehmanniae Radix exerted wound healing effect on human skin fibroblast cell line Hs27 via the activation of transformation growth factor (TGF-β) pathway and promoting extracellular matrix (ECM) deposition. Phytomedicine 2012, 20, 9-16, doi:10.1016/j.phymed.2012.09.006. - Luessen, H.; de Leeuw, B.J.; Langemeÿer, M.W.E.; Boer, A.; Verhoef, J.C.; Junginger, H.E. Mucoadhesive Polymers in Peroral Peptide Drug Delivery. VI. Carbomer and Chitosan Improve the Intestinal Absorption of the Peptide Drug Buserelin In Vivo. Pharm. Res. 1996, 13, 1668-1672, doi:10.1023/A:1016488623022. - Liu, R.; Zhang, L.; Lan, X.; Li, L.; Zhang, T.-T.; Sun, J.-H.; Du, G.-H. Protection by borneol on cortical neurons against oxygenglucose deprivation/reperfusion: Involvement of anti-oxidation and anti-inflammation through nuclear transcription factor κappaB signaling pathway. Neuroscience 2011, 176, 408-419, doi:10.1016/j.neuroscience.2010.11.029. - Blass, S.C.; Goost, H.; Tolba, R.H.; Stoffel-Wagner, B.; Kabir, K.; Burger, C.; Stehle, P.; Ellinger, S. Time to wound closure in trauma patients with disorders in wound healing is shortened by supplements containing antioxidant micronutrients and glutamine: A PRCT. Clin. Nutr. 2012, 31, 469-475, doi:10.1016/j.clnu.2012.01.002. - Thakur, R.; Jain, N.; Pathak, R.; Sandhu, S. Practices In Wound Healing Studies Of Plants. Evid. Based. Complement. Alternat. Med. 2011, 2011, 438056, doi:10.1155/2011/438056. - Rafiq, M.; Hussain, T.; Abid, S.; Nazir, A.; Masood, R. Development of sodium alginate/PVA antibacterial nanofibers by the incorporation of essential oils. Mater. Res. Express. 2018, 5, 35007, doi:10.1088/2053-1591/aab0b4. - Vasile, B.S.; Birca, A.C.; Musat, M.C.; Holban, A.M. Wound Dressings Coated with Silver Nanoparticles and Essential Oils for The Management of Wound Infections. Materials 2020, 13, 1682, doi:10.3390/ma13071682. - Karavasili, C.; Tsongas, K.; Andreadis, I.I.; Andriotis, E.G.; Papachristou, E.T.; Papi, R.M.; Tzetzis, D.; Fatouros, D.G. Physicomechanical and finite element analysis evaluation of 3D printable alginate-methylcellulose inks for wound healing applications. Carbohydr. Polym. 2020, 247, 116666, doi:10.1016/j.carbpol.2020.116666. - Li, T.-T.; Li, J.; Zhang, Y.; Huo, J.-L.; Liu, S.; Shiu, B.-C.; Lin, J.-H.; Lou, C.-W. A study on artemisia argyi oil/sodium alginate/PVA nanofibrous membranes: Micro-structure, breathability, moisture permeability, and antibacterial efficacy. J. Mater. Res. Technol. 2020, 9, 13450-13458, doi:10.1016/j.jmrt.2020.09.075. - Lamarra, J.; Calienni, M.N.; Rivero, S.; Pinotti, A. Electrospun nanofibers of poly(vinyl alcohol) and chitosan-based emulsions functionalized with cabreuva essential oil. Int. J. Biol. Macromol. 2020, 160, 307-318, doi:10.1016/j.ijbiomac.2020.05.096. - Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H.; Imran, M. Encapsulation of Cardamom Essential Oil in Chitosan Nano-composites: In-vitro Efficacy on Antibiotic-Resistant Bacterial Pathogens and Cytotoxicity Studies. Front. Microbiol. 2016, 7, 1580. Available online: https://www.frontiersin.org/article/10.3389/fmicb.2016.01580 (accessed on 11 November 2020). - Cremar, L.; Gutierrez, J.; Martinez, J.; Materon, L.; Gilkerson, R.; Xu, F.; Lozano, K. Development of antimicrobial chitosan based nanofiber dressings for wound healing applications. Nanomed. J. 2018, 5, 6-14, doi:10.22038/nmj.2018.05.002. - Rieger, K.A.; Schiffman, J.D. Electrospinning an essential oil: Cinnamaldehyde enhances the antimicrobial efficacy of chitosan/poly(ethylene oxide) nanofibers. Carbohydr. Polym. 2014, 113, 561-568, doi:10.1016/j.carbpol.2014.06.075. - Singh, S.; Gupta, A.; Sharma, D.; Gupta, B. Dextran based herbal nanobiocomposite membranes for scar free wound healing. Int. J. Biol. Macromol. 2018, 113, 227-239, doi:10.1016/j.ijbiomac.2018.02.097. - Mouro, C.; Simões, M.; Gouveia, I.C. Emulsion Electrospun Fiber Mats of PCL/PVA/Chitosan and Eugenol for Wound Dressing Applications. Adv. Polym. Technol. 2019, 2019, 9859506, doi:10.1155/2019/9859506. - Ardekani, N.T.; Khorram, M.; Zomorodian, K.; Yazdanpanah, S.; Veisi, H.; Veisi, H. Evaluation of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with Zataria multiflora essential oil as potential wound dressing. Int. J. Biol. Macromol. 2019, 125, 743-750, doi:10.1016/j.ijbiomac.2018.12.085. - Ge, Y.; Tang, J.; Fu, H.; Fu, Y. Terpinen-4-ol liposomes-incorporated chitosan/polyethylene oxide electrospun nanofibrous film ameliorates the external microenvironment of healing cutaneous wounds. J. Appl. Polym. Sci. 2021, 138, 49670, doi:10.1002/app.49670. - Barzegar, S.; Zare, M.R.; Shojaei, F.; Zareshahrabadi, Z.; Koohi-Hosseinabadi, O.; Saharkhiz, M.J.; Iraji, A.; Zomorodian, K.; Khorram, M. Core-shell chitosan/PVA-based nanofibrous scaffolds loaded with Satureja mutica or Oliveria decumbens essential oils as enhanced antimicrobial wound dressing. Int. J. Pharm. 2021, 120288, doi:10.1016/j.ijpharm.2021.120288. - Yan, X.; Yu, M.; Ramakrishna, S.; Russell, S.J.; Long, Y.-Z. Advances in portable electrospinning devices for in situ delivery of personalized wound care. Nanoscale 2019, 11, 19166-19178, doi:10.1039/C9NR02802A. - Dursun, N.; Liman, N.; Ozyazgan, I.; Gunes, I.; Saraymen, R. Role of thymus oil in burn wound healing. J. Burn Care Rehabil. 2003, 24, 395-399, doi:10.1097/01.BCR.0000095513.67541.0F. - de Oliveira, M.L.M.; Bezerra, B.M.O.; Leite, L.O.; Girão, V.C.C.; Nunes-Pinheiro, D.C.S. Topical continuous use of Lippia sidoides Cham. essential oil induces cutaneous inflammatory response, but does not delay wound healing process. J. Ethnopharmacol. 2014, 153, 283-289, doi:10.1016/j.jep.2014.02.030. - Gunal, M.; Heper, A.; Zaloglu, N. The Effects of Topical Carvacrol Application on Wound Healing Process in Male Rats. Pharmacogn. J. 2014, 6, 10-13, doi:10.5530/pj.2014.3.2. - Süntar, I.; Akkol, E.K.; Tosun, A.; Keleş, H. Comparative pharmacological and phytochemical investigation on the woundhealing effects of the frequently used essential oils. J. Essent. Oil Res. 2014, 26, 41-49, doi:10.1080/10412905.2013.820672. - Costa, M.F.; Durço, A.O.; Rabelo, T.K.; Barreto, R.d.S.; Guimarães, A.G. Effects of Carvacrol, Thymol and essential oils containing such monoterpenes on wound healing: A systematic review. J. Pharm. Pharmacol. 2019, 71, 141-155, doi:10.1111/jphp.13054. - Buyana, B.; Aderibigbe, B.A.; Ndinteh, D.T.; Fonkui, Y.T.; Kumar, P. Alginate-pluronic topical gels loaded with thymol, norfloxacin and ZnO nanoparticles as potential wound dressings. J. Drug Deliv. Sci. Technol. 2020, 60, 101960, doi:10.1016/j.jddst.2020.101960. - Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76-94, doi:10.3109/1040841X.2013.763219. - Riella, K.R.; Marinho, R.R.; Santos, J.S.; Pereira-Filho, R.N.; Cardoso, J.C.; Albuquerque-Junior, R.L.C.; Thomazzi, S.M. Antiinflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. J. Ethnopharmacol. 2012, 143, 656-663, doi:10.1016/j.jep.2012.07.028. 146. Guimarães, A.G.; Quintans, J.S.S.; Quintans-Júnior, L.J. Monoterpenes with Analgesic Activity—A Systematic Review. Phyther. Res. 2013, 27, 1-15, doi:10.1002/ptr.4686. - Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091-20112, doi:10.3390/molecules191220091. - Sami, D.G.; Abdellatif, A.; Azzazy, H.M.E. Turmeric/oregano formulations for treatment of diabetic ulcer wounds. Drug Dev. Ind. Pharm. 2020, 46, 1613-1621, doi:10.1080/03639045.2020.1811305. - Berechet, M.D.; Gaidau, C.; Miletic, A.; Pilic, B.; Râpă, M.; Stanca, M.; Ditu, L.-M.; Constantinescu, R.; Lazea-Stoyanova, A. Bioactive Properties of Nanofibres Based on Concentrated Collagen Hydrolysate Loaded with Thyme and Oregano Essential Oils. Materials 2020, 13, 1618, doi:10.3390/ma13071618. - Wasupalli, G.K.; Verma, D. Molecular interactions in self-assembled nano-structures of chitosan-sodium alginate based polyelectrolyte complexes. Int. J. Biol. Macromol. 2018, 114, 10-17, doi:10.1016/j.ijbiomac.2018.03.075. - Kenawy, E.; Omer, A.M.; Tamer, T.M.; Elmeligy, M.A.; Eldin, M.S.M. Fabrication of biodegradable gelatin/chitosan/cinnamaldehyde crosslinked membranes for antibacterial wound dressing applications. Int. J. Biol. Macromol. 2019, 139, 440-448, doi:10.1016/j.ijbiomac.2019.07.191. - Liakos, I.; Rizzello, L.; Hajiali, H.; Brunetti, V.; Carzino, R.; Pompa, P.P.; Athanassiou, A.; Mele, E. Fibrous wound dressings encapsulating essential oils as natural antimicrobial agents. J. Mater. Chem. B 2015, 3, 1583-1589, doi:10.1039/C4TB01974A. - Ge, Y.; Tang, J.; Fu, H.; Fu, Y.; Wu, Y. Characteristics Controlled-release and Antimicrobial Properties of Tea Tree Oil Liposomesincorporated Chitosan-based Electrospun Nanofiber Mats. Fibers Polym. 2019, 20, 698-708, doi:10.1007/s12221-019-1092-1. - Lam, N.S.K.; Long, X.X.; Griffin, R.C.; Chen, M.-K.; Doery, J.C.G. Can the tea tree oil (Australian native plant: Melaleuca alternifolia Cheel) be an alternative treatment for human demodicosis on skin? Parasitology 2018, 145, 1510-1520. https://doi.org/doi:10.1017/S0031182018000495. - Rahman, S.M.A.; Nabi, M.N.; Van, T.C.; Suara, K.; Jafari, M.; Dowell, A.; Islam, M.A.; Marchese, A.J.; Tryner, J.; Hossain, M.F.; et al. Performance and Combustion Characteristics Analysis of Multi-Cylinder CI Engine Using Essential Oil Blends. Energies 2018, 11, 738, doi:10.3390/en11040738. - Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50-62, doi:10.1128/CMR.19.1.50-62.2006. - Sadri, M.; Arab-Sorkhi, S.; Vatani, H.; Bagheri-Pebdeni, A. New wound dressing polymeric nanofiber containing green tea extract prepared by electrospinning method. Fibers Polym. 2015, 16, 1742-1750, doi:10.1007/s12221-015-5297-7. - Sugumar, S.; Ghosh, V.; Nirmala, M.J.; Mukherjee, A.; Chandrasekaran, N. Ultrasonic emulsification of eucalyptus oil nanoemulsion: Antibacterial activity against Staphylococcus aureus and wound healing activity in Wistar rats. Ultrason. Sonochem. 2014, 21, 1044-1049, doi:10.1016/j.ultsonch.2013.10.021. - Sugumar, S.; Mukherjee, A.; Chandrasekaran, N. Eucalyptus oil nanoemulsion-impregnated chitosan film: Antibacterial effects against a clinical pathogen, Staphylococcus aureus, in vitro. Int. J. Nanomed. 2015, 10 (Suppl. 1), 67-75, doi:10.2147/IJN.S79982. - Yousefi, I.; Pakravan, M.; Rahimi, H.; Bahador, A.; Farshadzadeh, Z.; Haririan, I. An investigation of electrospun Henna leaves extract-loaded chitosan based nanofibrous mats for skin tissue engineering. Mater. Sci. Eng. C 2017, 75, 433-444, doi:10.1016/j.msec.2017.02.076. - Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of Lavender essential oil. Phyther. Res. 2002, 16, 301-308, doi:10.1002/ptr.1103. - Cuttle, L.; Pearn, J.; McMillan, J.R.; Kimble, R.M. A review of first aid treatments for burn injuries. Burns 2009, 35, 768-775, doi:10.1016/j.burns.2008.10.011. - Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Athanassiou, A.; Mele, E. Alginate-lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B 2016, 4, 1686- 1695, doi:10.1039/C5TB02174J. - Alam, P.; Ansari, M.J.; Anwer, M.K.; Raish, M.; Kamal, Y.K.T.; Shakeel, F. Wound healing effects of nanoemulsion containing clove essential oil. Artif. Cells Nanomed. Biotechnol. 2017, 45, 591-597, doi:10.3109/21691401.2016.1163716.
